Your Partner in Delivering Accessible, Highly Efficient Cell Therapy for All
What we offer
ImmunSkills SA unveils a comprehensive one-stop solution in cell therapy, offering immune cells that are not only engineered to withstand tumor microenvironments but are also primed for large-scale cultivation. With a platform that is meticulously designed for flexibility, we can effortlessly and efficiently incorporate any CAR provided by our clients, ensuring precise targeting against a broad spectrum of solid cancers. We stand as your steadfast partner from the initial stages at the laboratory bench all the way through to market release. Our responsibility encompasses everything from small-scale production for animal testing to large-scale manufacturing for both clinical trials and market supply, all while maintaining compliance with cGMP standards. This allows you to channel your focus exclusively on CAR development, leaving the rest of the intricate processes in our capable hands.
Our technology
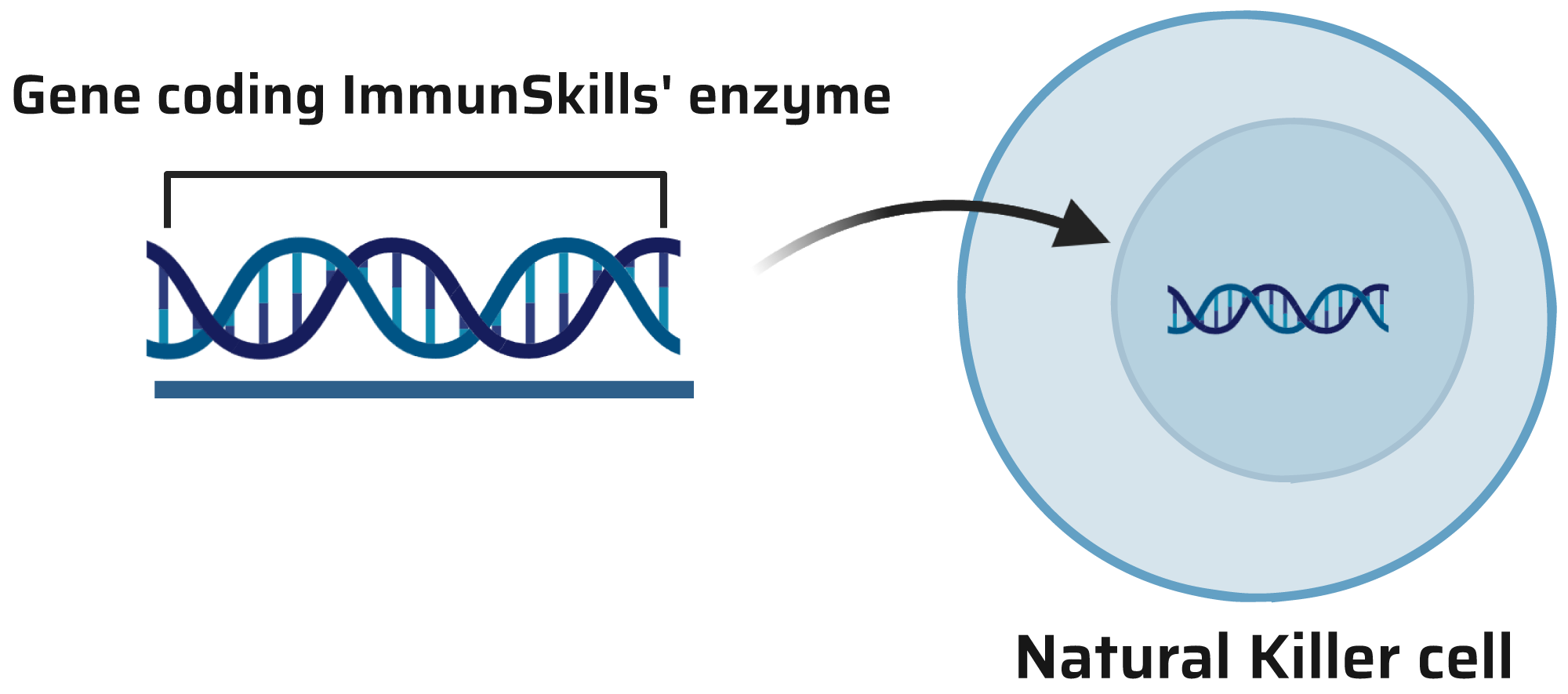
Enhanced Cells with Enzyme Technology
ImmunSkills SA has meticulously developed cells empowered with a specialized enzyme, significantly enhancing their efficiency within the Tumor Microenvironment (TME). This enzyme acts as a shield, allowing the cells to thrive within TME’s challenging conditions. Our state-of-the-art tools for CAR transfection into these fortified cells are highly efficient, facilitating seamless CAR integration and function within the cell. These enhanced cells, available in R&D and GMP format, are ready for use in a variety of research and development applications, supporting all stages of preclinical development, including animal models, as well as clinical and market production.
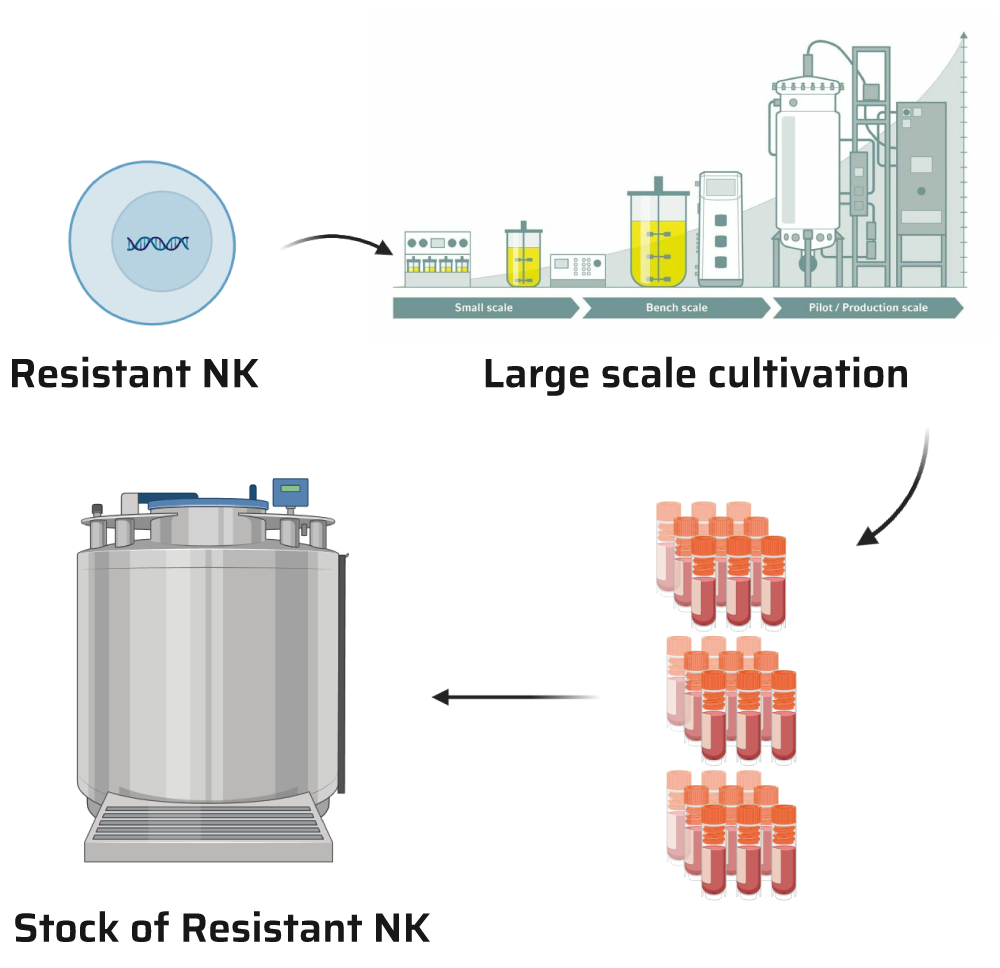
We’ve optimized our cells for large-scale cultivation, with the capability to grow up to 200 litres. This allows us to produce and store substantial stock quantities right on our premises. When needed, these cells can be swiftly thawed and utilized in crafting cell therapies tailored for our customers

Our platform
Scalable Process with your CAR
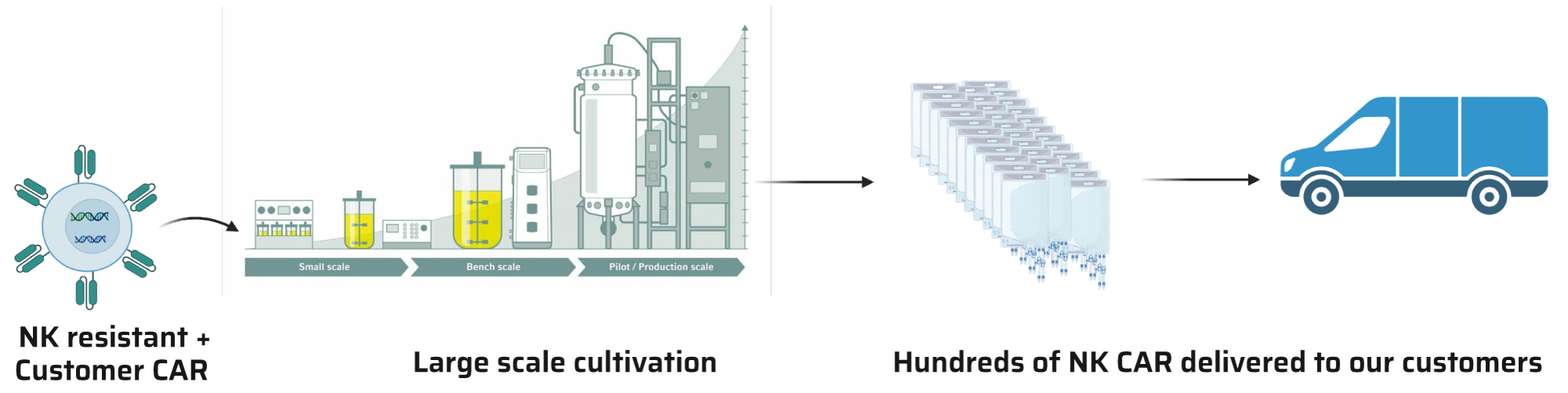
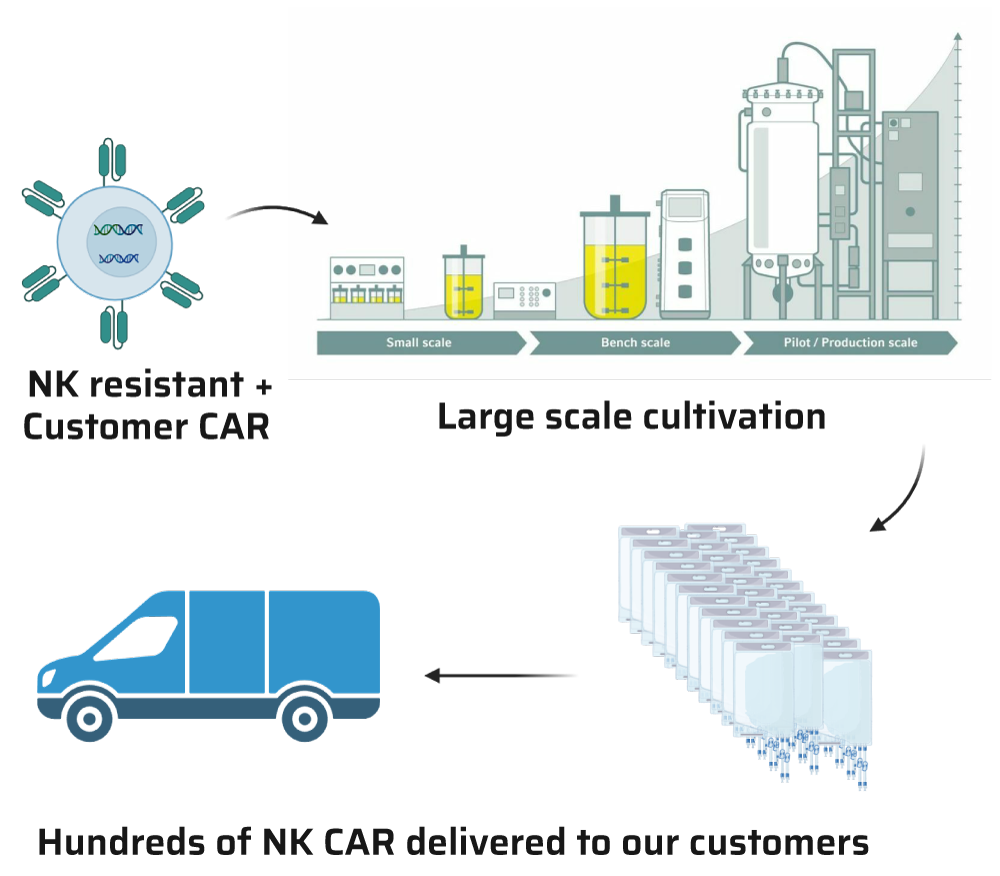
Our groundbreaking technology also includes a scalable process designed to support the growth of our enhanced cells bearing your CAR at high densities. This adaptable process, available in both R&D and cGMP formats, supports both preliminary research and production for clinical trials, ensuring a supply of efficiently functioning cells for every development stage. Whether you’re conducting initial research or preparing for clinical trials and market production, our scalable process meets your needs by supporting high-density cell growth at each development stage.

Our clients supply their specific CAR recognition area. We seamlessly integrate this with our stimulation domain, guaranteeing optimal cellular activity and superior therapeutic outcomes. After introducing the CAR into our cells, we thoroughly assess its performance. We then culture it to the desired scale, ensuring delivery of a top-quality end product.
Our Services
Providing tailored solutions to meet your needs, our team is dedicated to delivering excellence in service.




